Epigenetic mechanisms play an important role in the determination of hematopoietic stem and progenitor cell (HSPC) fate through the modification of chromatin architecture. Polycomb repressive complex 2 (PRC2) is an indispensable epigenetic modifier that catalyzes histone 3 lysine 27 tri-methylation (H3K27me3) repressive mark through its catalytic subunit EZH2. PRC2 is divided into two independent complexes, PRC2.1 and PRC2.2, based on the specific interacting accessory proteins. PRC2.1 comprises one of the three polycomb-like proteins (PCL1/PHF1, PCL2/MTF2, or PCL3/PHF19), as well as one of the two recently identified accessory proteins, EPOP or PALI1. However, PRC2.2 contains either JARID2 or AEBP2 as co-factors. These accessory proteins aid in the recruitment of the PRC2 complexes to different sites in the genome to catalyze H3K27me3. Recent studies performed on mouse embryonic stem cells showed that PRC2.1 and PRC2.2 are involved in distinct lineage specification and commitment through their specific co-factors. However, the functional differences between PRC2.1 and PRC2.2 in HSPC differentiation and lineage commitment has not been thoroughly characterized.
To compare the complete loss of PRC2 versus PRC2.2-specific loss of function in normal hematopoiesis, we generated Mx1-Cre: Jarid2fl/fl and Mx1-Cre: Ezh2fl/fl mouse strains. Two-month old Mx1-Cre(+) control, Mx1-Cre(+): Jarid2fl/fl, and Mx1-Cre(+): Ezh2fl/fl mice were injected with six doses of polyinosinic:polycytidylic acid (pIpC) to promote conditional deletion of Jarid2 and Ezh2. Two months post-pIpC injection, whole bone marrow (WBM) cells were used to perform competitive transplantation (5x10 5 CD45.2 test WBM plus 5x10 5 CD45.1 competitor WBM cells). Jarid2-KO primary recipients showed the highest CD45.2 peripheral blood (PB) engraftment (~80%), and the highest tri-lineage (myeloid 80%, B cell 70%, T cell 80%) chimerism compared to the control (myeloid 50%, B cell 45%, T cell 25%) and Ezh2-KO (myeloid 65%, B cell 5%, T cell 5%) recipients. Ezh2-KO recipients showed the lowest CD45.2 PB engraftment (~20%), and were deficient for PB lymphoid cell production. Jarid2-KO recipients showed the highest bone marrow (BM) engraftment (~ 90%), while Ezh2-KO recipients showed significant BM lymphoid engraftment (B cell 55% and T cell 40%) inverse to what was observed in the PB (B cell 5% and T cell 5%). This suggested that complete loss of PRC2 function promotes blockage in the migration of mature lymphoid cells from BM to the PB. Jarid2-KO and Ezh2-KO recipients showed significant reductions in phenotypically defined HSC cell number and frequency compared to control recipients suggesting that PRC2.2 function through its co-factor Jarid2 to maintain HSC pool.
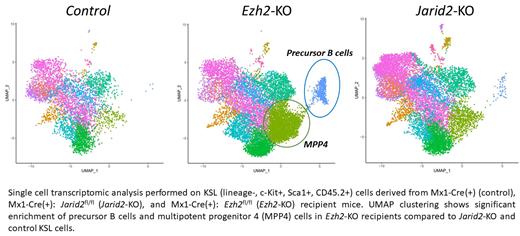
To further investigate the functional effect of both genes' loss of function at the molecular level, we performed single cell transcriptomic analysis from KSL (lineage-, c-Kit+, Sca1+, CD45.2+) cells sorted from the aforementioned recipients. Based on transcriptomic analysis, Ezh2 loss of function promoted an increase in MPP4 and precursor B cell populations in the BM of recipient mice. However, no significant changes was observed between control and Jarid2-KO clusters. Analysis showed that the Mtf2 co-factor has the highest expression level throughout the whole KSL clusters compared to other PRC2.1 and PRC2.2 accessory proteins. These results suggested that Mtf2-associated PRC2.1 is necessary to restrict MPP4 self-renewal and to repress B cell priming in HSPCs. Although no significant change was observed in the HSC cluster between Jarid2-KO and Ezh2-KO genotypes, Jarid2 and Ezh2 loss of functions showed significant differential gene expression in the HSC population when compared to each other and to the control suggesting that Jarid2-associated PRC2.2 regulates a unique molecular program in HSCs independently from PRC2.1. Combined with our functional data, these results suggested that PRC2.2 restricts HSC-specific gene expression in non-self-renewing progenitor cells through its co-factor Jarid2; while PRC2.1 restricts MPP4 fate and B cell priming through its co-factor Mtf2. Ongoing studies are aimed to delineate the distinct molecular programs regulated by PRC2.1 and PRC2.2 in HSPCs using CRISPR-Cas9 sgRNA lentiviral system targeting each of the PRC2.1 and PRC2.2 accessory proteins independently and validation in lineage-specific functional assays.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal